Get Started
REAL CHEM students reach their course one of two ways. Identify which one applies to you:
- A single sign-on (SSO) in your school website, like Canvas, Blackboard, or Brightspace. A single sign-on link never asks you to sign in — it just takes you directly to the course. If your instructor uses a REAL CHEM SSO link, that’s all you need!
- An enrollment URL. When you follow an enrollment URL you’re asked to sign in to REAL CHEM’s platform Torus, or create an account if you don’t have one yet. This is called “Direct Delivery.”
Direct Delivery students
Enroll in OLI Torus course (one time)
Follow the invitation link, create an account, confirm the email address, sign in to OLI Torus, and access the course materials. See the detailed steps below the video.
- Follow the invitation URL provided by your instructor / school and click Create an Account on the right side of the page or sign in with an existing account. Use a valid email.
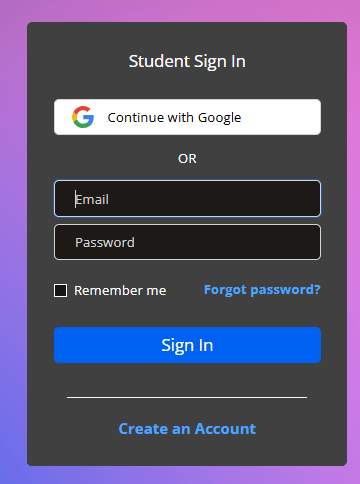
View the confirmation message: To continue, check your email for a confirmation email. If you don’t receive this email, check your Spam folder or verify that the entered email is correct.
The tab may be closed.
Note: If you do not receive an email confirmation, please email oli-help@cmu.edu for assistance. Don’t forget to check SPAM or other filtered folders! - Click the Confirm Email button from the email confirmation in that inbox with the subject “Confirm your email address” from Open Learning Initiative to confirm the email and go to the sign-in page.
- Sign in with your new account on the right side of the page. Note: Sign in even if the sign-in is labeled instructor sign-in.
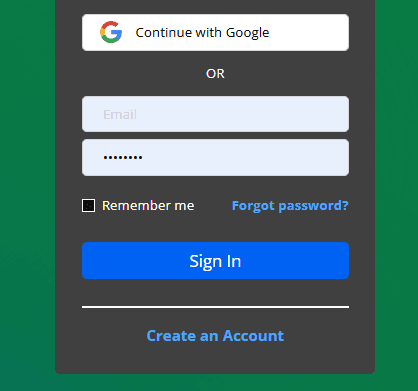
- Click to check “I’m Not a Robot” and Enroll.
- Click the start course message as indicated by the Welcome course landing page.
Make a note of your OLI Torus Learner Account ID (email address) and Password!
Access OLI Torus after enrollment in the course section
Return to your REAL CHEM course by signing in at at https://proton.oli.cmu.edu/ with the existing learner account.





